Programmable nucleases
The study of molecular mechanisms of hereditary multigene diseases is a complex, largely unsolved problem. This is due to a large variety of genes, a large number and variety of mutation that are directly related to the development of the disease, the presence of a large spectrum of single-nucleotide polymorphisms of the genome and other modifying factors, many of which are personalized. All this leads to great difficulties in the development of new tools for the treatment of hereditary diseases. Such diseases include many neurodegenerative and cardiovascular pathologies, in particular, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, hypertrophic cardiomyopathy, long QT syndrome, etc. For a successful search for target molecules and the initial screening of potential drug compounds, it is necessary to create adequate models that allow in vitro experiments on relevant types of human cells. Human induced pluripotent stem cells can be differentiated in wide range of derivatives, including cardiomyocytes and neurons of various types. The use of these cells in conjunction with CRISPR-mediated genome editing systems opens the broadest possible prospects for creating cellular models of hereditary pathologies. Using CRISPR/Cas9-mediated homologous recombination in AAVS1 locus we obtained clones of iPSCs which contain transgenes intended for doxycycline-dependent expression of two forms of programmed AsCpf1(Cas12a) nuclease, which have two different PAM (protospacer adjacent motif) consensus — 5′-TTTV-3 ‘ and 5’- TYCV-3 ‘. These iPSC lines can be used to create knockout and transgenic lines, as well as cell models of diseases associated with single nucleotide polymorphisms.
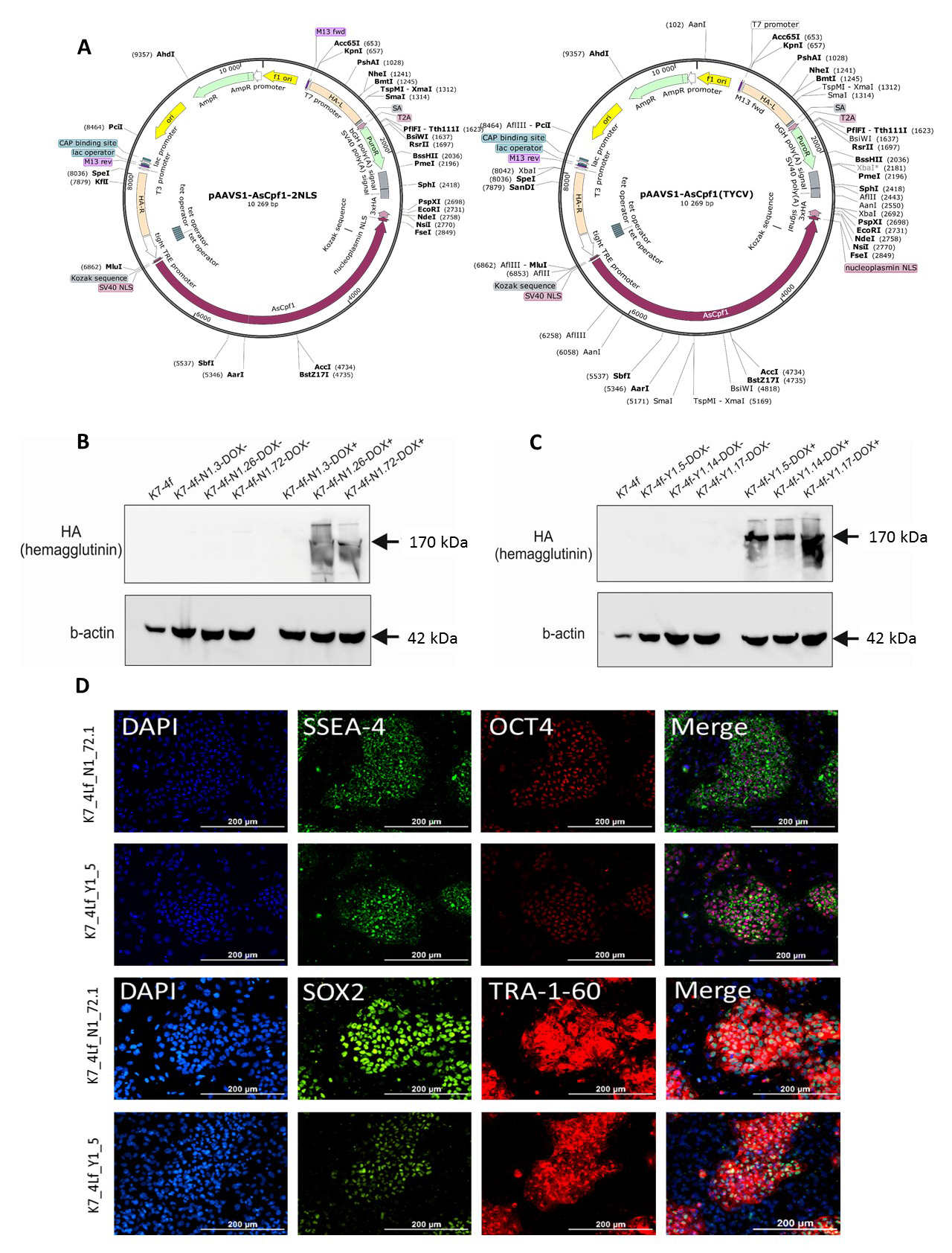
The study of molecular mechanisms of hereditary multigene diseases is a complex, largely unsolved problem. This is due to a large variety of genes, a large number and variety of mutation that are directly related to the development of the disease, the presence of a large spectrum of single-nucleotide polymorphisms of the genome and other modifying factors, many of which are personalized. All this leads to great difficulties in the development of new tools for the treatment of hereditary diseases. Such diseases include many neurodegenerative and cardiovascular pathologies, in particular, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, hypertrophic cardiomyopathy, long QT syndrome, etc. For a successful search for target molecules and the initial screening of potential drug compounds, it is necessary to create adequate models that allow in vitro experiments on relevant types of human cells. Human induced pluripotent stem cells can be differentiated in wide range of derivatives, including cardiomyocytes and neurons of various types. The use of these cells in conjunction with CRISPR-mediated genome editing systems opens the broadest possible prospects for creating cellular models of hereditary pathologies. Using CRISPR/Cas9-mediated homologous recombination in AAVS1 locus we obtained clones of iPSCs which contain transgenes intended for doxycycline-dependent expression of two forms of programmed AsCpf1(Cas12a) nuclease, which have two different PAM (protospacer adjacent motif) consensus — 5′-TTTV-3 ‘ and 5’- TYCV-3 ‘. These iPSC lines can be used to create knockout and transgenic lines, as well as cell models of diseases associated with single nucleotide polymorphisms.
