Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is one of the most common cardiovascular diseases and is characterized by abnormal thickness of left ventricular wall and interventricular septum, diastolic dysfunction, progressive heart failure, and a high risk of arrhythmias and sudden death. Inherited form of the disease is caused predominately by mutations in genes encoding for sarcomere-associated proteins. However, no effective methods to prevent HCM progression have been developed so far. Patient-specific iPSC-derived cardiomyocytes can afford new opportunities in unraveling HCM pathogenic mechanisms. Using episomal vectors, we generated iPSC lines from peripheral blood mononuclear cells of two HCM patients carrying mutations with unknown clinical significance in disease-associated genes – p.M659I in MYH7 ( https://hpscreg.eu/cell-line/ICGi019-A , https://hpscreg.eu/cell-line/ICGi019-B ) and p.N515del in MYBPC3 ( https://hpscreg.eu/cell-line/ICGi029-A ).
The iPSC lines can become a tool for clarifying the roles of the mutations in HCM pathogenesis, for studying molecular mechanisms of the disease, and for testing drugs and new therapy methods. In addition, we generated an iPSC line from a patient carrying a heterozygous p.E510Q mutation in HADHA encoding for long-chain 3-hydroxyacyl-CoA dehydrogenase that participates in mitochondrial fatty acid β-oxidation ( https://hpscreg.eu/cell-line/ICGi028-A ). This iPSC line can be used for generating cardiomyocytes that may represent an HCM case caused by metabolic impairments.
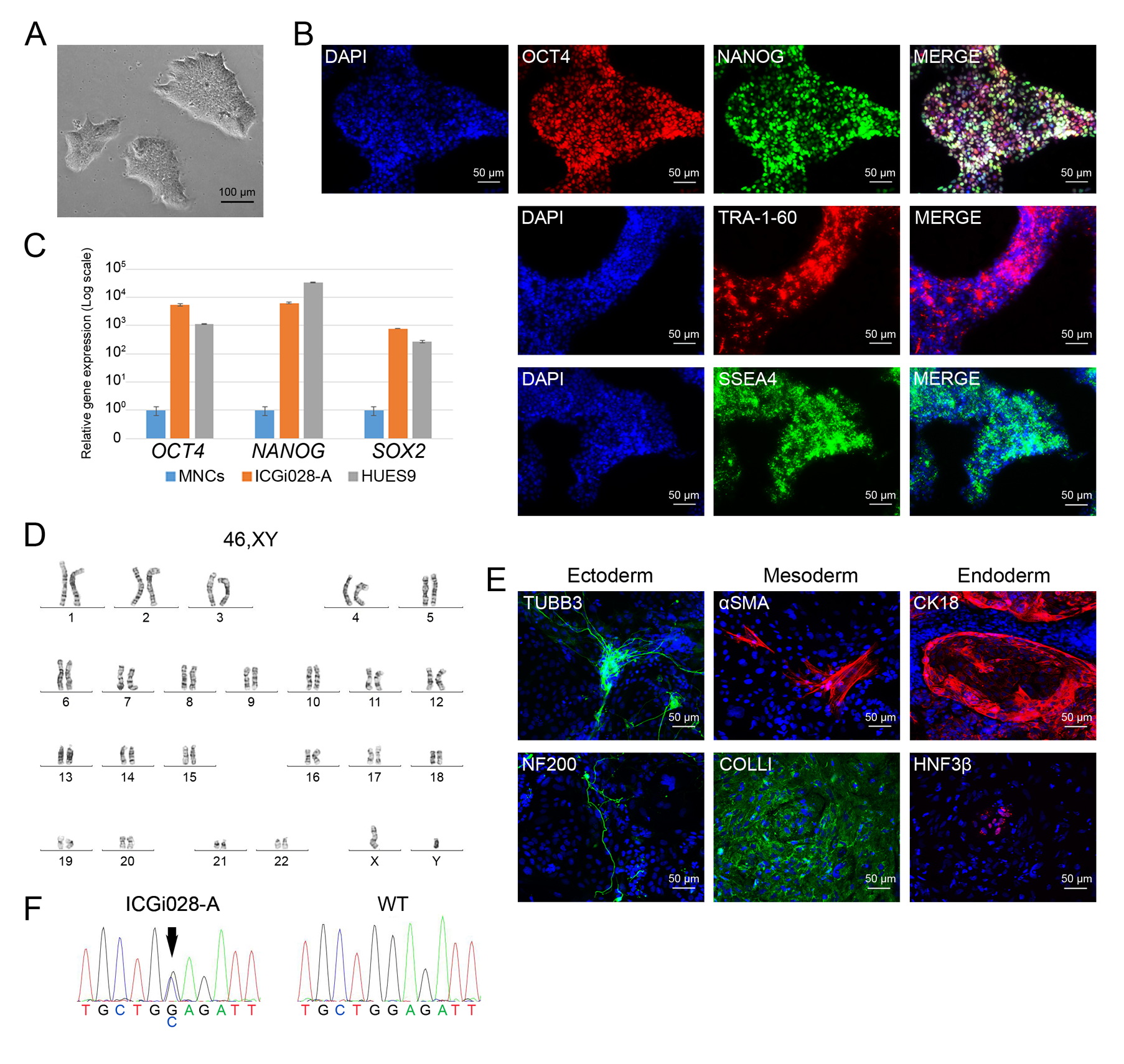
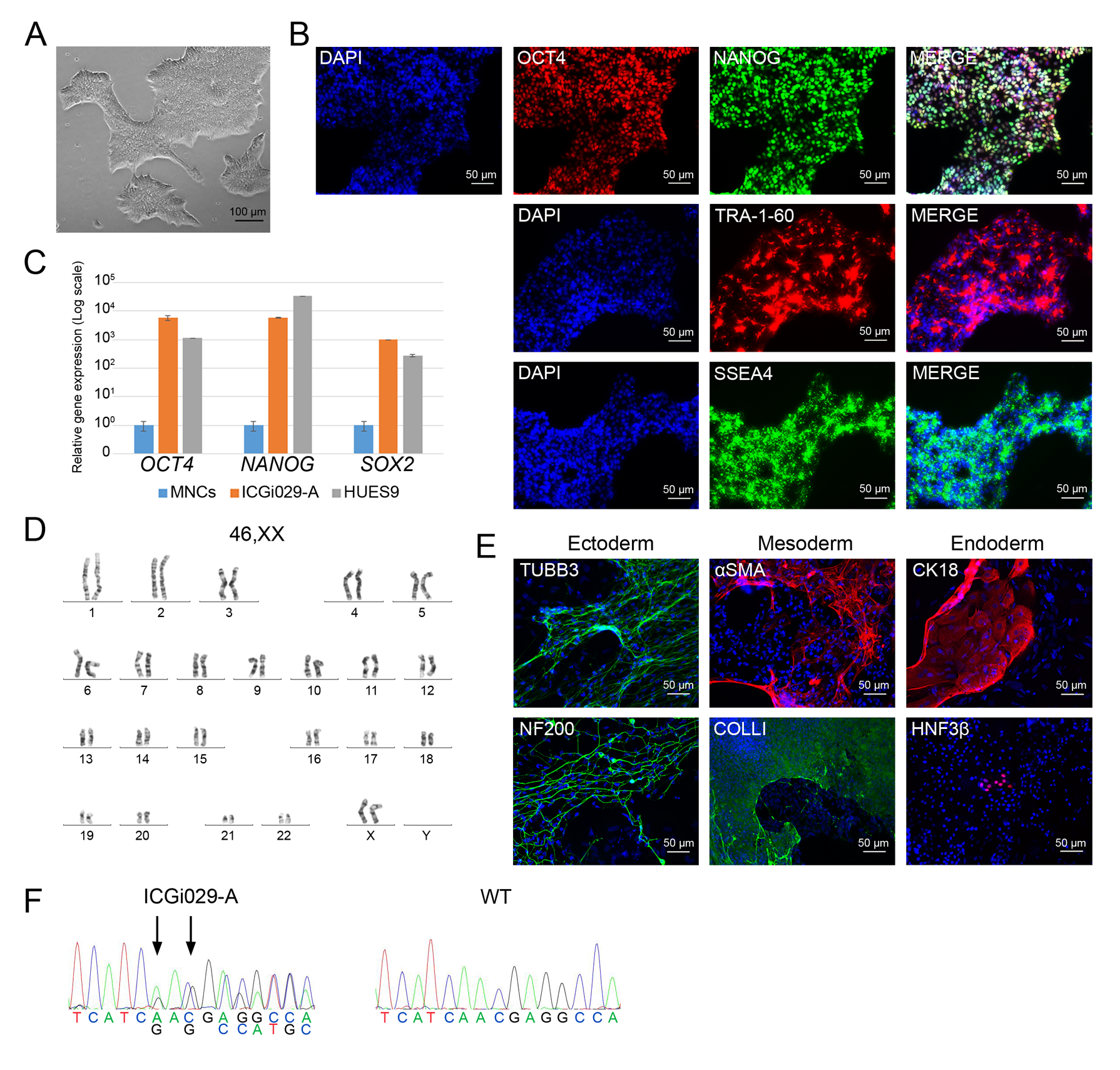
Hypertrophic cardiomyopathy (HCM) is one of the most common cardiovascular diseases and is characterized by abnormal thickness of left ventricular wall and interventricular septum, diastolic dysfunction, progressive heart failure, and a high risk of arrhythmias and sudden death. Inherited form of the disease is caused predominately by mutations in genes encoding for sarcomere-associated proteins. However, no effective methods to prevent HCM progression have been developed so far. Patient-specific iPSC-derived cardiomyocytes can afford new opportunities in unraveling HCM pathogenic mechanisms. Using episomal vectors, we generated iPSC lines from peripheral blood mononuclear cells of two HCM patients carrying mutations with unknown clinical significance in disease-associated genes – p.M659I in MYH7 ( https://hpscreg.eu/cell-line/ICGi019-A , https://hpscreg.eu/cell-line/ICGi019-B ) and p.N515del in MYBPC3 ( https://hpscreg.eu/cell-line/ICGi029-A ).
The iPSC lines can become a tool for clarifying the roles of the mutations in HCM pathogenesis, for studying molecular mechanisms of the disease, and for testing drugs and new therapy methods. In addition, we generated an iPSC line from a patient carrying a heterozygous p.E510Q mutation in HADHA encoding for long-chain 3-hydroxyacyl-CoA dehydrogenase that participates in mitochondrial fatty acid β-oxidation ( https://hpscreg.eu/cell-line/ICGi028-A ). This iPSC line can be used for generating cardiomyocytes that may represent an HCM case caused by metabolic impairments.
