Hypoxia model
Hypoxia-inducible factors (HIFs) are transcription factors that play an important role in the adaptive response to hypoxic conditions. HIFs are stabilized in hypoxia but degraded in normoxia. HIF-2α subunit is involved in the regulation of transcription factors, controlling self-renewal of human pluripotent stem cells, embryonic development of the cardiovascular system and the regulation of angiogenesis by transcriptional activation of angiogenic cascades in physiological and pathological processes. Currently, modulation of HIF-2α expression is considered to be a promising strategy for ischemic and cancer disease treatment. However, the problem of choosing the optimal methods of effective regulation of HIF-2α still remains.
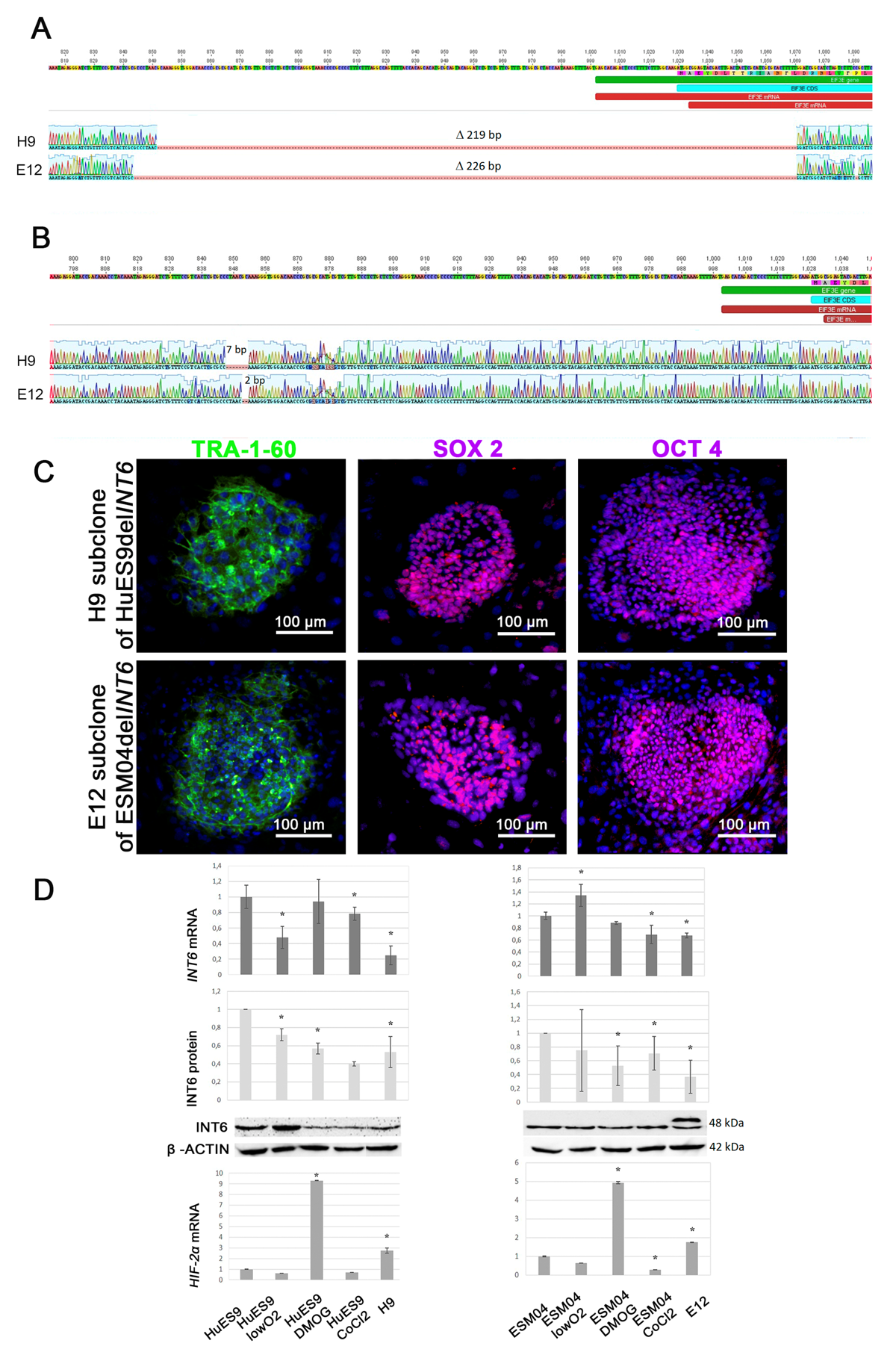
We have obtained genetically modified human embryonic stem cells with increased expression of HIF-2α under atmospheric oxygen conditions. The approach used is based on a CRISPR/Cas9-mediated deletion within INT6 gene coding HIF-2α inhibitor protein. A study of the resulting genetically modified human embryonic stem cells will contribute to an understanding of the connection between hypoxia and pluripotency. Obtaining endothelial derivatives of pluripotent stem cells with increased expression of HIF-2α and enhanced regenerative potential may become the basis for the development of promising strategies for the treatment of ischemic diseases.
Hypoxia-inducible factors (HIFs) are transcription factors that play an important role in the adaptive response to hypoxic conditions. HIFs are stabilized in hypoxia but degraded in normoxia. HIF-2α subunit is involved in the regulation of transcription factors, controlling self-renewal of human pluripotent stem cells, embryonic development of the cardiovascular system and the regulation of angiogenesis by transcriptional activation of angiogenic cascades in physiological and pathological processes. Currently, modulation of HIF-2α expression is considered to be a promising strategy for ischemic and cancer disease treatment. However, the problem of choosing the optimal methods of effective regulation of HIF-2α still remains.
We have obtained genetically modified human embryonic stem cells with increased expression of HIF-2α under atmospheric oxygen conditions. The approach used is based on a CRISPR/Cas9-mediated deletion within INT6 gene coding HIF-2α inhibitor protein. A study of the resulting genetically modified human embryonic stem cells will contribute to an understanding of the connection between hypoxia and pluripotency. Obtaining endothelial derivatives of pluripotent stem cells with increased expression of HIF-2α and enhanced regenerative potential may become the basis for the development of promising strategies for the treatment of ischemic diseases.
